Metal-Dependent Asynchronicity of Concerted Proton–Electron Transfer to a High-Valent Copper (III) Complex and Its Nickel (III) Analogue #448
Authors
Katherine J. Fisher, H. Ray Kelly, Claire C. Cody, Cristina Decavoli, Brandon Q. Mercado, Jennifer L. Troiano, Robert H. Crabtree, Victor S. Batista, Gary W. Brudvig
Abstract
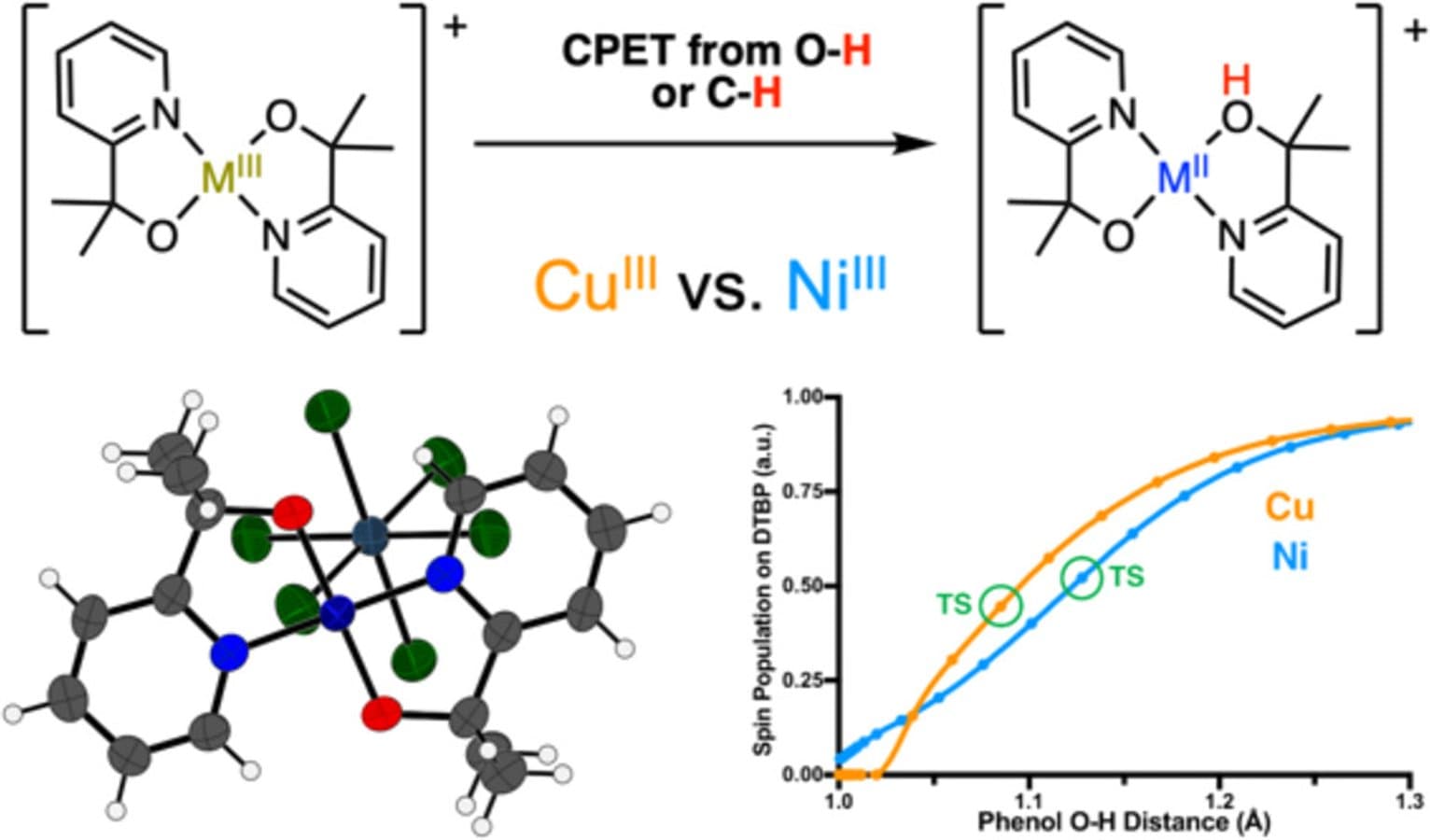
A high-valent formally copper(III) complex, [Cu(pyalk)2]+(2) (pyalk = 2-(2′-pyridyl)-2-propanolate), is isolated and characterized by a variety of physical methods, including X-ray crystallography and DFT computational modeling. Complex 2 is found to undergo fast proton-coupled electron transfer with phenol and hydrocarbon substrates, resulting in the reduction of the metal center and protonation of the pyalk ligand. Analysis of kinetic data for the reaction of 2 with both types of substrates suggests that 2 reacts through a concerted proton–electron transfer (CPET) pathway. Thermodynamic analysis indicates that the O–H bond formed during CPET by 2 has a high bond dissociation enthalpy of 103 kcal/mol, consistent with the fast reactivity of 2 as compared to its isostructural nickel(III) analogue, [Ni(pyalk)2]+ (4). Compared to 4, 2 reacts 5–10 times faster with phenol and hydrocarbon substrates and has an O–H BDE ∼6 kcal/mol higher than 4. Further analysis suggests that 4 may undergo CPET through a more basic asynchronous pathway than 2, which may cause the CPET rate with 4 to be much faster than expected from the Bell–Evans–Polanyi principle.